Get Healthy!
- Posted May 22, 2023
Another Death, More Cases of Vision Loss Linked to Tainted Eye Drops
Cases of vision loss and deaths are mounting in an investigation into eye drops contaminated with a rare strain of a drug-resistant bacteria.
In all, four people have died, with one new death now being reported by the U.S. Centers for Disease Control and Prevention. Meanwhile, a total of 14 people have experienced vision loss, with six new cases reported last week.
Infections have now been reported in 81 people living in 18 states. Four cases have involved people who have had to have their eyeballs surgically removed.
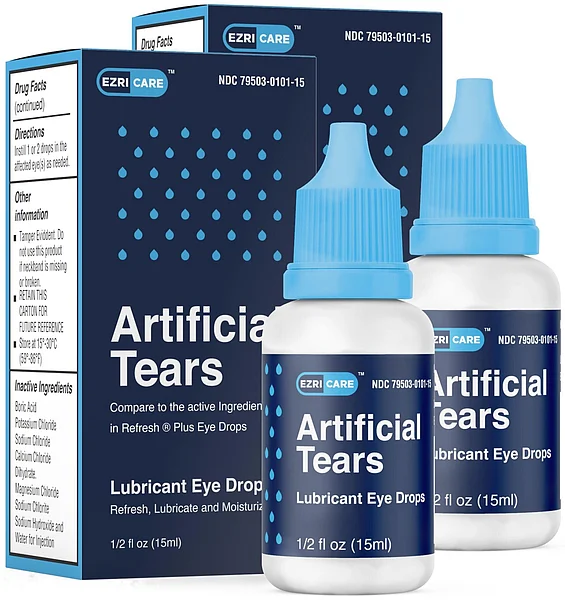
The cases involve 10 brands of eye drops, most commonly EzriCare Artificial Tears. Global Pharma Healthcare's Artificial Tears Lubricant Eye Drops were first recalled in February. The drops were distributed by EzriCare and Delsam Pharma.
"Patients who have used EzriCare or Delsam Pharma's artificial tears and who have signs or symptoms of an eye infection should seek medical care immediately,"the CDC said in its latest update.
Pseudomonas aeruginosa is the bacteria infecting these patients. It's a strain never before seen in the United States, CNN reported.
Among the newly identified patients, many had either recalled using the eyedrops or lived in long-term care facilities in which others were infected with the bacteria.
The bacteria can spread to those who haven't used the drops, the CDC noted.
"The bacteria can spread when one patient carrying the bacteria exposes another patient, or when patients touch common items or when healthcare workers transmit the germs, which is why infection control, like hand hygiene, is so important,"the CDC previously told CNN.
Signs of infection can include yellow, green or clear discharge from the eye. They can also include eye pain or discomfort, redness of the eye or eyelid, a feeling that something is in the eye, light sensitivity and blurry vision.
The U.S. Food and Drug Administration inspected the Global Pharma facility for 11 days in mid-February. In a report that was released afterwards, the agency said that the plant's manufacturing process "lacked assurance of product sterility"for batches made between December 2020 and April 2022.
The FDA has urged people to stop using the recalled eye drops.
More information
The Indiana Department of Health describes Pseudomonas aeruginosa.
SOURCE: CNN

